MannKind Corp. (Nasdaq: MNKD) stock doubled Wednesday after its inhaled diabetes treatment, Afrezza, was recommended for approval by a Food and Drug Administration (FDA) advisory committee.
In a unanimous 14 to 0 thumbs-up, the group voted Afrezza should be approved for type 2 diabetes, the more common form of the blood sugar disease. In a near-united nod, the group voted 13 to 1 in favor of Afrezza's approval for type 1 diabetes.
"We are pleased with the Advisory Committee's approval recommendation in support of Afrezza, and we appreciate the thoroughness of their review," Alfred Mann, MannKind Corp. chairman and chief executive officer, said in a statement. "We look forward to working with the FDA as they complete their evaluation of Afrezza. Diabetes is a major health problem in the United States, and we are committed to bring Afrezza to the many patients who might benefit from this novel product."
The next move is the FDA's, which has until April 15 to a make a decision. While it isn't required to follow the panel's advice, a strong endorsement does carry some weight with the FDA.
A go-ahead from the strict government agency, however, isn't guaranteed by any means. And Afrezza concerns linger.
Worries that Afrezza may affect lung function surfaced in a March 28 FDA report. Additionally, hesitations were heightened over missing data from a type 1 diabetes study.
Indeed, some FDA advisory panel members who backed Afrezza approval communicated reservations about possible safety risks. But, they said MannKind proved the drug works. The advisory group added that the benefit as an alternative to insulin injections outweighed their concerns and could be clearly started on the medicine's label.
To understand how this could benefit MannKind, here are some details on how this treatment works...
MannKind's (Nasdaq: MNKD) Afrezza
A rapid-acting insulin powder, Afrezza comes in a single-use cartridge designed to be inhaled at the start of a meal. Afrezza dissolves immediately upon inhalation to the deep lung and delivers insulin quickly to the bloodstream.
According to MannKind, patients using the drug can achieve peak insulin levels in 12 to 15 minutes. That compares to the 90 to 150 minutes or more wait time after patients inject regular insulin.
Based in Valencia, Calif., MannKind has been working some seven years to get approval for this pioneering diabetes treatment. Rejected twice by the FDA, most recently in 2011 after the company elected to switch inhalers during the review process, MannKind hopes the third time is the charm.
If approved, Afrezza would be MannKind's first drug to hit the market. According to Bloomberg,it could generate $583 million in sales by 2018. That's likely just the start...
The type 2 diabetes drug market constitutes a sizable chunk of the overall diabetes drug market. The segment is expected to grow from $26 billion in 2011 to $50 billion in 2021 in developed markets including the United States, Japan, and Europe.
Diabetes is a chronic condition in which the body either doesn't make ample amounts of insulin to break down sugars in foods or it can't use insulin efficiently. It causes a host of ailments, including blindness, strokes, and heart disease. Demand for diabetes treatments are soaring along with growing global obesity. In fact, obesity and diabetes prevalence have reached epidemic proportions, according to the World Health Organization.
In the United States alone, 26 million adults and children suffer with diabetes. According to the American Diabetes Association, 1.9 million American are diagnosed with the disease every year. If current trends continue, an estimated one in three Americans adults are expected to have diabetes by 2050.
MannKind (Nasdaq: MNKD) Faces Big-Name Competition
Small-cap MannKind will have some stiff competition from big market players if its diabetes treatment is approved.
Afrezza will compete with Eli Lily & Co.'s (NYSE: ELI) Humalog and Novo Nordisk's (NYSE: NVO) Novolog. In 2013, Humalog's sales tally was $2.6 billion and Novolog's was $3 billion. Moreover, many patients with diabetes begin treatment with Teva Pharmaceuticals Industries Ltd.'s (NYSE: TEVA) metformin, an oral pill better known as Glucophage.
But in Afrezza's court, both Lily's and Novo Nordisk's treatments are injectable. And, metformin loses its effectiveness over time.
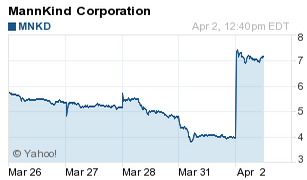
MNKD investors remain guarded. Shares plunged about 32% over the last five sessions as investors awaited news from the FDA.
MNKD got a boost from MLV & Co. Wednesday when the New York-based investment bank upgraded its price target on the stock to $11 from $9. Meanwhile, Cowen and Company cut its price target to $5 from $5.50. Plus, option speculators have increased MNKD put (bearish) wagers.
Shortly before noon, MNKD shares had given up some gains but were still up a sweet 80% at $7.22.
There are huge profits to be made in the biotech sector. But before you try investing in biotech stocks, you need to know this one thing...
Related Articles:
- Bloomberg:
FDA Panel Backs MannKind Corp's Diabetes Drug Afreaza - Associated Press:
Ahead of the Bell: MannKind Shares Soar - MarketWatch:
MannKind Soars on Drug Recommendation; Apollo Slumps


