It's known as the "blockbuster," and it's the Holy Grail in the world of medicine - a Big Pharma drug that generates big yearly and lifetime sales because it targets a big opportunity in maladies that afflict millions of people.
Indeed, the three biggest blockbusters of all time target high cholesterol (Lipitor), inflammatory diseases (Humira), and digestive afflictions (Nexium) and have pulled in a combined $350 billion - and counting.
But what about people suffering from rare diseases? Here we're talking about such afflictions as cystic fibrosis, acute myeloid leukemia, multiple myeloma, renal cell carcinoma, ovarian cancer, Duchenne muscular dystrophy, glioma, and pancreatic cancer.
Many of these diseases are genetic, appear early in life, and are tough to treat. Indeed, while more than 7,000 rare diseases have been identified, about 95% have no approved therapies.
As many as 30 million Americans live with some type of rare disease, says the National Center for Advancing Translation Sciences Genetic and Rare Disease Information Center (GARD).
Compare that with diabetes - which all by itself affects more than 30 million Americans.
Given that drug firms and biotechs alike are striving for that Holy Grail blockbuster - and thus are targeting the maladies with the biggest number of sufferers - what's the outlook for rare-disease sufferers? Will drug companies develop drugs to ease their suffering?
Thankfully, the answer increasingly is "yes."
Here in America, Washington understands the need for these "orphan drugs" targeting rare diseases - and actually created financial incentives to encourage drugmakers to develop them. The "Orphan Drug Act of 1983" offers a seven-year window of tax reductions and the exclusive right to market a drug for a particular rare disease.
(You'll find somewhat similar regulatory designations in Europe, Japan, Singapore, and Australia to encourage the development of drugs to treat orphan diseases.)
The incentives are helping. North America leads the world in orphan drugs. Here in the United States, the number of orphan drugs coming to market has zoomed from a single digit in 1983 to 40 the next year to 121 in 2007.
To date, more than 600 orphan drugs have been approved by the U.S. Food and Drug Administration (FDA).
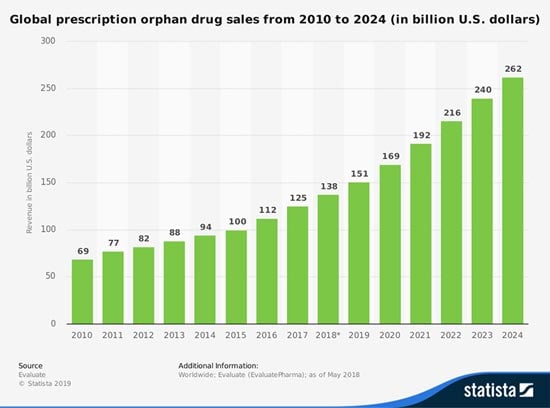
And the global market for orphan drugs targeting rare diseases is growing at double the rate of the non-orphan market. In 2017, $125 billion worth of rare disease drugs were sold - a total that will grow at a compound annual growth rate (CAGR) of 11.3% between now and 2024, when it reaches $262 billion.
That total would account for 21.8% of the $1.2 billion prescription drug market.
More needs to be done. And that "more" will create a massive investment portal - provided you pick the best-positioned companies.
That's why today, I'm going to show you a revved-up leader in the specialty-drugs market whose shares could double in under three years...[mmpazkzone name="in-story" network="9794" site="307044" id="137008" type="4"]
Untangling Specialty Medicine
It's one of those odd paradoxes of life that while each rare disease market is small, the underlying challenges are often quite complex. For drugmakers looking to create specialty/orphan therapeutics, the profit potential can be quite massive.
The makers of orphan drugs generally benefit from higher profit margins as an incentive to navigate the challenge of clinical trials and FDA approval.
The firm I have in mind for you is tackling cystic fibrosis (CF) - a genetic disorder that hampers the breathing and digestion of about 30,000 Americans. While there are treatments that help mitigate some of the symptoms, there is no cure.
Many different mutations to a single gene can cause CF. But the bottom line is that the protein cells that need to push out water don't function correctly.
Because of this defective protein, bodily fluids like sweat, digestive fluids, or mucus that are usually thin become abnormally thick.
Mucus, for example, is normally thin enough to clean the lungs by capturing foreign particles and then moving those invaders up the windpipe. In CF sufferers, the mucus is so thick that it pools in the lungs - trapping contaminants and causing lung-damaging infections.
The pancreas, liver, kidneys, and other organs are similarly afflicted. People with cystic fibrosis on average live to be only 42 to 50 years old - way below the U.S. average of 78.9 years.
That's where Vertex Pharmaceuticals Inc. (NASDAQ: VRTX) comes in. Founded in 1989 in Cambridge, Mass., Vertex was the first company to ever receive FDA approval for a CF drug that treats the disease's underlying causes - and not just the symptoms.
The drug, ivacaftor or Kalydeco, acts as a "booster" that helps cells activate the protein that is supposed to push out water - improving lung function by 10%. Unfortunately, this drug only works for 4% to 5% of cases, as it targets only specific mutations.
Then, in 2015, the company received FDA approval for a combination drug that works for about 50% of all cystic fibrosis cases.
Three years later, the FDA approved another Vertex combination drug targeting even more mutations.
And just last year, the FDA approved a Vertex drug called Trikafta that's said to be effective in treating 90% of CF cases.
That's a game changer, and studies show people on Vertex's treatments are 33% less likely to need hospitalization and a whopping 71% less likely to need a lung transplant.
With all this in mind, let's run it through my filters for finding true tech wealth.
Tech Wealth Rule No. 1: Great Companies Have Great Operations
These are well-run firms with top-notch leaders.
A true key to Vertex's amazing progress is Dr. Reshma Kewalramani, the company's president and CEO. Kewalramani joined the company in 2017 as the chief medical officer and executive VP of global-medicines development and spearheaded the development and approval for the 2018 and 2019 drugs.
She's also been instrumental in shepherding some of Vertex's other drugs from the lab into clinical trials.
Kewalramani amassed this skillset during 12 years in research-and-development (R&D) work at pharma giant Amgen Inc. (NASDAQ: AMGN), where she won numerous awards.
Tech Wealth Rule No. 2: Separate the Signal from the Noise
To create real wealth, you have to stay focused and ignore the messy hype that dominates headlines in order to identify companies that have rock-solid fundamentals.
Earnings drive growth. But right now, most companies are pulling guidance - leaving investors flying blind. Not Vertex.
Back in April, Vertex actually boosted its full-year 2020 guidance. Now the company is expected to boost sales by 37% and earnings by a staggering 65% this year, says Zacks Investment Research.
Zacks actually gives Vertex its top rating - saying it's a No. 1 "Strong Buy."
Vertex shares are up nearly 25% year to date.
Tech Wealth Rule No. 3: Ride the Unstoppable Trends
Look for stocks in red-hot sectors because they offer the best chance for life-changing gains.
Today, any news about COVID-19 immediately captures headlines. Meanwhile, politicians from both parties agree that we need treatments, tests, and vaccines against this pandemic - preferably yesterday.
In other words, this pandemic has been a good reminder that cutting-edge medical science is crucial.
In fact, according to Global Market Insights, the world's biotech market is set to grow by 8.3% a year until 2025 when it will be worth $729 billion. Vertex will be a big beneficiary - and is likely going to grow faster than the market average.
Tech Wealth Rule No. 4: Focus on Growth
Companies that have the strongest growth rates almost always offer the highest stock returns.
Vertex is sitting on a big pipeline: It has eight drugs in clinical trials and two more still in preclinical development.
The firm has also licensed out four other drugs to big pharmaceuticals like German giant Merck & Co. Inc. (NYSE: MRK). This means Vertex gets paid as the drugs succeed, but it doesn't have to spend money developing them itself.
And of course, with four cystic fibrosis drugs approved since 2012, there's plenty of money coming in the door already.
Over the last three years alone, Vertex has grown sales by 31% a year. With its impressive pipeline, the firm can keep that up for years to come.
Tech Wealth Rule No. 5: Target Stocks That Can Double Your Money
This is where we look at the firm's earnings growth and see how long it will take to double profits. By doing that, we can figure out how long on average it should take for the stock to roughly double.
After pouring through the financials in detail, I'm projecting that earnings per share will grow at an average of 35% a year.
And that's a conservative estimate, as the real number over the last three years has been more than double that rate.
Now we use what I call my doubling calculator. Mathematicians call it the "Rule of 72."
Let's divide the compound profit growth rate of 35% into the number 72.
That tells us Vertex stock is set to give us a double in just over two years. I believe the firm will remain at the forefront of treating CF and other genetic disorders for many years to come, giving this winner a very long runway.
So if you're looking to build wealth over the long haul, this biotech deserves to be on your personal "watch list" - and probably in your portfolio.
In the meantime, don't forget to watch my latest presentation for this can't-miss opportunity...
You see, the country may be reopening, but the race for a coronavirus vaccine is still in full swing. The FDA has just issued a policy that will expedite the vaccine approval process exponentially, and it could very well benefit this tiny company.
In fact, they are already planning to ship their first million doses by year's end, and folks like you could bank a huge gain when all is said and done.
How did a company 1/200th the size of Pfizer develop what could be the most lucrative drug in history? Click here to find out more...
Follow Money Morning on Facebook and Twitter.
About the Author
Michael A. Robinson is a 36-year Silicon Valley veteran and one of the top tech and biotech financial analysts working today. That's because, as a consultant, senior adviser, and board member for Silicon Valley venture capital firms, Michael enjoys privileged access to pioneering CEOs, scientists, and high-profile players. And he brings this entire world of Silicon Valley "insiders" right to you...
- He was one of five people involved in early meetings for the $160 billion "cloud" computing phenomenon.
- He was there as Lee Iacocca and Roger Smith, the CEOs of Chrysler and GM, led the robotics revolution that saved the U.S. automotive industry.
- As cyber-security was becoming a focus of national security, Michael was with Dave DeWalt, the CEO of McAfee, right before Intel acquired his company for $7.8 billion.
This all means the entire world is constantly seeking Michael's insight.
In addition to being a regular guest and panelist on CNBC and Fox Business, he is also a Pulitzer Prize-nominated writer and reporter. His first book Overdrawn: The Bailout of American Savings warned people about the coming financial collapse - years before the word "bailout" became a household word.
Silicon Valley defense publications vie for his analysis. He's worked for Defense Media Network and Signal Magazine, as well as The New York Times, American Enterprise, and The Wall Street Journal.
And even with decades of experience, Michael believes there has never been a moment in time quite like this.
Right now, medical breakthroughs that once took years to develop are moving at a record speed. And that means we are going to see highly lucrative biotech investment opportunities come in fast and furious.
To help you navigate the historic opportunity in biotech, Michael launched the Bio-Tech Profit Alliance.
His other publications include: Strategic Tech Investor, The Nova-X Report, Bio-Technology Profit Alliance and Nexus-9 Network.



