Tekmira Pharmaceuticals Corp. (Nasdaq: TKMR) stock surged some 20% to $17.23 Friday after an update on its Ebola treatment.
The U.S. Food and Drug Administration modified its clinical hold status on its experimental Ebola treatment to partial hold, enabling it for potential use in humans infected with the virus.
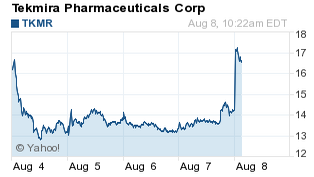 TKMR stock was halted late Thursday ahead of the news. The stock was up 6.65% on volume of 2.1 million shares in Thursday's regular session, and soared another 6.87% to $15.25 after hours.
TKMR stock was halted late Thursday ahead of the news. The stock was up 6.65% on volume of 2.1 million shares in Thursday's regular session, and soared another 6.87% to $15.25 after hours.
"We are pleased that the FDA has considered the risk-reward of TKM-Ebola for infected patients," Dr. Mark Murray, Tekmira's chief executive officer and president said in a statement. "We have been closely watching the Ebola virus outbreak and its consequences, and we are willing to assist with any responsible use of TKM-Ebola. The foresight shown by the FDA removes one potential roadblock to doing so. This current outbreak underscores the critical need for effective therapeutic agents to treat the Ebola virus. We recognize the heightened urgency of this situation, and are carefully evaluating options for use of our investigational drug within accepted clinical and regulatory protocols."
Tekmira stock jumped 40% last week as the worst-ever Ebola outbreak accelerated. Investors piled in even as human tests of TKM-Ebola were put on hold last month due to safety concerns.
TKMR stock, however, swooned Monday after CNN reported two ailing American missionary workers suffering with Ebola were treated, with some success, by an investigational drug from a competing biotech company: privately held Mapp Biopharmaceuticals.
Yet amid the recent Ebola outbreak, which has sickened 1,700 and killed nearly 1,000 people in West Africa, the race is on to find a treatment. And Tekimira's TKM-Ebola does show promise.
Presently, no approved treatment or vaccine for Ebola exists. There is not even one that has passed the first phase of safety trials in human volunteers.
The World Health Organization (WHO) has declared the Ebola outbreak an international health emergency, heightening the urgency to find a treatment. The current Ebola crisis is on pace to sicken more people than all other previous outbreaks of the disease combined, according to the Centers for Disease Control and Prevention (CDC).
"It is clear to say that the disease is uncontained and out of control in West Africa," Ken Isaacs, vice president of Programs and Government Relations at the relief organization Samaritan's Purse, said at a congressional hearing Thursday. "The international response to the disease has been a failure."
On Wednesday, WHO said it would convene a meeting of medical ethics experts next week to consider the implication of making experimental Ebola drug widely available.
Indeed, "the only way to discover whether new therapies are effective is to test them during an epidemics," Dr. Jeremy Farrar, director of the Wellcome Trust, Dr. David Heymann, head and senior fellow of the Chatham House Centre on Global Health Security, and Dr. Peter Piot, director of the London School of Hygiene and Tropical Medicine, wrote in an op-ed piece in Thursday's Wall Street Journal.
The doctors added that African countries, where the current outbreaks are occurring, should have the same opportunity to receive in-trial treatments as developed countries.
Trial Drugs from a Trio of Companies Ready to Go
The U.S. Agency for International Development is spending $14.5 million to combat the Ebola outbreak. It has sent a disaster response team to West Africa to assist workers. The work includes sending tens of thousands of protective suits for healthcare workers.
In addition, the CDC is working to open more treatment centers and expand proper Ebola testing. Equipment has already been sent and training has been prepared in Ghana, predicted to be the next country hit with the disease.
Tekmira's Ebola treatment is one of three worldwide that have shown especially promising results in monkeys, but it is unproven in humans.
Sarepta Therapeutics Inc. (Nasdaq: SRPT) is another biotech company prepared to provide an Ebola drug.
"We have a drug that could be deployed and shipped," Sarepta's CEO Chris Garabedian told Barron's. He said any use of the drug would require waivers and approval from the Department of Defense, which paid for the development of the drug, as well as the Food and Drug Administration. The Sarepeta Ebola drug showed survival rates of 60% to 80% in primates, versus a zero percent survival rate among untreated animals
The Cambridge, Mass., drug company halted development on the drug two years ago. Still, SRPT says it has enough in supply to treat a few dozen patients. Shares jumped 1.59% to $20.20 Friday morning.
BioCryst Pharmaceuticals Inc. (Nasdaq: BCRX) is yet another biotech with a pipeline drug that has shown promise fighting Ebola, as well as viral infections such as Marburg and Rift Valley Fever.
In March, the Durham, N.C.-based small cap reported that its under-development drug, BCX4430, protected rodents against Marbug and Ebola infections.
BCRX shares soared 6.28% to $13.71 Friday. Shares are up a meteoric 78.82% year to date.
Today's top investor story: Even though its stock climbed 103% in just a few days, this hyped IPO is one investors should avoid...
Related Articles:


